Team:
Krzysztof Dariusz Pluta, PhD – head of
the Laboratory of Tissue Engineering
Joanna Agata Motyl, PhD
Agnieszka Wencel, PhD Eng.
Małgorzata Jakubowska, PhD Eng.
Monika Joanna Wiśniewska, MSc
MAIN RESEARCH TOPICS
The liver has a great regenerative potential, however, in case of a complete loss of its function, the only effective therapy is organ transplantation. Unfortunately, due to the lack of donors, about 10% of patients waiting for liver transplantation die annually. Therefore, the scientists have been searching for the new alternatives to this type of treatment. The most promising are extracorporeal hybrid devices, which support diseased liver until the organ regeneration or transplantation can take place. The most advanced systems utilize hollow fiber bioreactors populated with hepatic cells, so-called bioartificial livers (BALs).
The main aim of our research is to develop the improved biological part of BAL. The best source of cells to use in such systems are human liver parenchymal cells - hepatocytes. However, they dedifferentiate very quickly in vitro and lose the ability to perform liver-specific functions. Therefore, the other sources of liver cells are used in BAL devices, for instance cells of animal origin, cancer cell lines, various types of stem cells or cells derived from induced pluripotent stem cells (iPSCs). In the best clinically tested BAL, ELAD, the human hepatoma line C3A was utilized. Unfortunately, the main disadvantage of these cells is the non-functional urea cycle. This could be the reason why this BAL failed in randomized clinical trials. Taking this into account, a new genetically modified (using lentiviral vectors based on HIV-1 genome) C3A cell line with restored urea cycle was developed in our Laboratory. These cells may be a better biological source to be used in bioartificial liver devices.
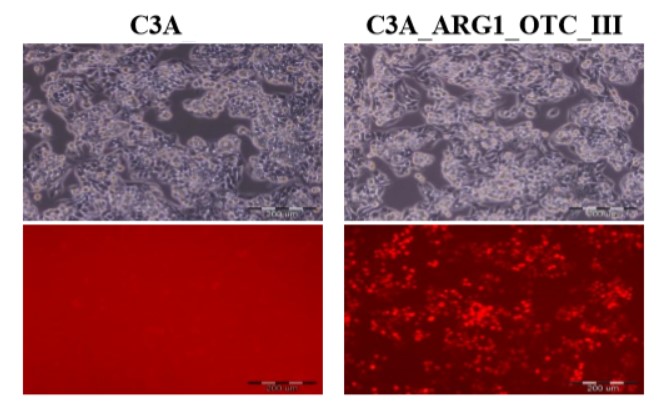 |
Human hepatoma cell line (C3A) and its genetically modified counterpart with restored urea cycle (C3A_ARG1_OTC_III). Photographs were taken using an Olympus IX71 fluorescence microscope. The upper panel shows phase-contrast transmitted images, while the lower panel shows red fluorescence of the marker mCherry protein under UV light in the UMN-G filter. Scale bar 200 µm.
Moreover, a new mechanical-enzymatic method for the isolation of human hepatocytes from the resected liver was created in our Laboratory (biological material is obtained from Medical University of Warsaw). The use of this technique enables faster cell isolation and higher cells viability when compared to the standard double perfusion method.
 |
Stages of mechanical-enzymatic method for liver cell isolation.
The Laboratory of Tissue Engineering also conducts research on long-term cultures of human hepatocytes. In order maintain isolated hepatocytes in the state of differentiation for a long time we are developing new culture substratum (for instance the substratum made of dried fibroblasts). Culture of human hepatocytes with other cell types also has an impact on the preservation of the specific liver functions. Therefore, in our Laboratory, genetic modifications of fibroblasts isolated from human skin were performed. We observed that the overproduction of the growth factors such as hepatocyte growth factor (HGF) or epidermal growth factor (EGF) by modified fibroblasts creates favorable conditions for the culture of parenchymal liver cells.
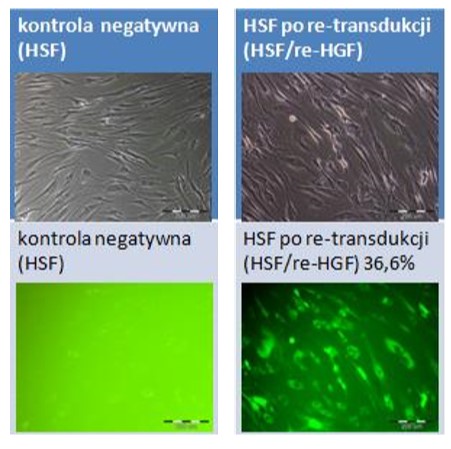
Fibroblasts isolated from human skin fibroblasts (HSF) and their genetically modified counterparts overproducing hepatocyte growth factor (HSF_HGF). Photographs were taken using an Olympus IX71 fluorescence microscope. The upper panel shows phase-contrast transmitted images, while the lower panel shows the green fluorescence of the marker EGFP protein in UV light in the UMN-M filter. Scale bar 200 µm.
Liver does not only consist of hepatocytes. Other cell types (including vascular endothelial cells, bile duct cells, specialized macrophages, stellate cells, and various progenitors) also have an important role in the structure and functions of the organ. Analysis of liver composition using flow cytometry is an original technique developed by our team and it could constitute in the future a unique prognostic and diagnostic method of liver diseases.
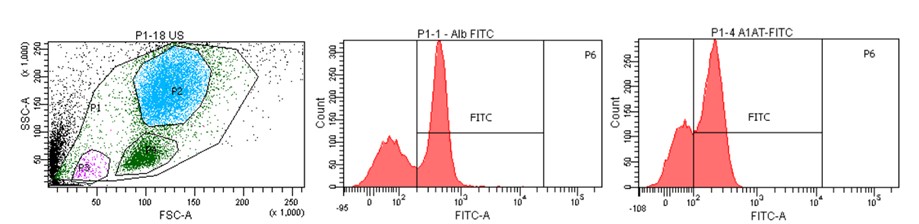
Cytometric image of the population of cells isolated from human liver with histograms showing characteristic liver markers (albumin, α-1-antitrypsin).
Equipment:
Our team use the Biological Laboratory, the facility fulfilling Biosafety Level 2 standard (BSL 2) and designed to work with Genetically Modified Microorganisms on the second level of biohazard (GMM 2). Our laboratory is equipped with class II laminar chambers, CO2 incubators, Olympus IX71 inverted fluorescence microscope, BD FACS Canto II flow cytometer, autoclaves, centrifuges, BIORAD thermocycler and small equipment for molecular biology.
Keywords:
tissue engineering, bioartificial liver, coculture with the feeder layer cells, lentiviral vectors, genetically modified cells, flow cytometry
Scientific projects:
1. Project Sonatina 3 from National Science Center nr 2019/32/C/NZ4/00455 "The importance of crosstalk between alpha-synuclein and sphingosine-1-phosphate-dependent signaling in experimental models of Parkinson's disease. Searching for novel therapeutic targets in synucleinopathies."
Head of the project: Joanna Agata Motyl, PhD
2. Project Miniatura 3 from National Science Center nr 2021/05/X/NZ3/01478 "Functional and genetic evaluation of modified C3A cells with restored urea cycle (C3A_AO) for construction of bioartificial liver and drug hepatotoxicity tests."
Head of the project: Agnieszka Wencel, PhD Eng.
Publications:
- Motyl JA, Gromadzka G, Czapski GA, Adamczyk A: SARS-CoV-2 Infection and Alpha-Synucleinopathies: Potential links and underlying mechanisms. International Journal of Molecular Sciences, 2024, 25(22):12079. doi: 10.3390/ijms252212079.
- Jakubowska M, Wiśniewska M, Wencel A, Wojciechowski C, Gora M, Dudek K, Chwojnowski A, Burzynska B, Pijanowska DG, Pluta KD: Hollow fiber bioreactor with genetically modified hepatic cells as a model of biologically active function block of the bioartificial liver. Biocybernetics and Biomedical Engineering, 2024, 44(1): 9-19. doi: 10.1016/j.bbe.2023.11.003.
- Motyl JA, Strosznajder JB, Wencel A, Strosznajder RP: Recent insights into the interplay of Alpha-Synuclein and Sphingolipid signaling in Parkinson's disease. International Journal of Molecular Sciences, 2021, 22(12): 6277. doi: 10.3390/ijms22126277.
- Pluta KD, Ciężkowska M, Wiśniewska M, Wencel A, Pijanowska DG: Cell-based clinical and experimental methods for assisting the function of impaired livers - present and future of liver support systems. Biocybernetics and Biomedical Engineering, 2021, 41(4): 1322-1346. doi: 10.1016/j.bbe.2021.06.005.
- Wencel A, Ciężkowska M, Wiśniewska M, Zakrzewska KE, Pijanowska DG, Pluta KD: Effects of genetically modified human skin fibroblasts, stably overexpressing hepatocyte growth factor, on hepatic functions of co-cultured C3A cells. Biotechnology and Bioengineering, 2021, 118(1): 72-81. doi: 10.1002/bit.27551.
- Pluta KD, Samluk A, Wencel A, Zakrzewska KE, Góra M, Burzyńska B, Ciężkowska M, Motyl J, Pijanowska DG: Genetically modified C3A cells with restored urea cycle for improved bioartificial liver. Biocybernetic and Biomedical Engineering, 2020, 40(1): 378-387. doi: 10.1016/j.bbe.2019.12.006.
- Ciężkowska M, Pluta KD: Kliniczne metody wspomagania niewydolnej wątroby. Postępy Biochemii, 2019, 65(3): 193-201. doi: 10.18388/pb.2019_269.
- Zakrzewska KE, Samluk A, Wencel A, Dudek K, Pijanowska DG, Pluta KD: Liver tissue fragments obtained from males are the most promising source of human hepatocytes for cell-based therapies – Flow cytometric analysis of albumin expression. PLoS ONE, 2017, 12(8): e0182846. doi: 10.1037/journal.pone.0182846.
- Wencel A, Zakrzewska KE, Samluk A, Noszczyk BH, Pijanowska DG, Pluta KD: Dried human skin fibroblasts as a new substratum for functional culture of hepatic cells. Acta Biochimica Polonica, 2017, 64(2): 357-363. doi: 10.18388/abp.2016_1481.
- Zakrzewska KE, Samluk A, Wierzbicki M, Jaworski S, Kutwin M, Sawosz E, Chwalibog A, Pijanowska DG, Pluta KD: Analysis of the cytotoxicity of carbon-based nanoparticles, diamond and graphite, in human glioblastoma and hepatoma cell lines. PloS ONE, 2015, 10(3): e0122579. doi: 10.1371/journal.pone.0122579.
- Zakrzewska KE, Samluk A, Pluta KD, Pijanowska DG: Evaluation of the effects antibiotics on cytotoxicity of EGFP and DsRed2 fluorescent proteins used for stable cell labeling. Acta Biochimica Polonica, 2014, 61(4): 809-813. doi: 10.18388/abp.2014_1850.
- Samluk A, Zakrzewska KE, Pluta KD: Generation of fluorescently labeled cell lines, C3A hepatoma cells and human adult skin fibroblasts, to study coculture models. Artificial Organs, 2013, 37(7): E123-30. doi: 10.1111/aor.12064.
- Pluta K, Kacprzak MM.: Use of HIV as a gene transfer vector. Acta Biochimica Polonica, 2009, 56(4): 531-595. doi: 10.18388/abp.2009_249.




















